Trogarzo (Ibalizumab)
Model: Trogarzo
Category: Pharmaceutical
Exhibitor: TAIMED BIOLOGICS INC.
Booth No: M104
Characteristic
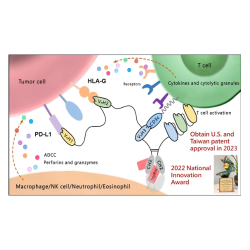
Trogarzo® is a long-acting monoclonal antibody which binds to domain 2 of the CD4 T cell receptors. It blocks viral entry into host cells while preserving normal immunologic function. Trogarzo® belongs to the CD4-directed post-attachment HIV-1 inhibitor class of antiretrovirals. In the United States, Trogarzo® is approved, in combination with other antiretroviral(s), for the treatment of HIV-1 infection in heavily treatment-experienced adults with multidrug resistant (MDR) HIV-1 infection failing their current antiretroviral regimen. Trogarzo® has been approved in the US since March 2018 and was the first intravenous agent approved for the treatment of HIV-1 infection. In 2019, Trogarzo® IV Push was also approved as a new administration route
Other Products
Products you may be interested in
Highest Rated Products