Drug Substance Manufacturing
Category: *CDMO
Exhibitor: AMARAN BIOTECHNOLOGY, INC.
Booth No: M726
Characteristic
Amaran Biotech’s teams have the technical expertise and knowledge of the necessary standards to execute the manufacturing of complex, high-value drug substances. We have a solid track record of successful delivery of a full spectrum of products with a special focus in the realm of immunotherapy.
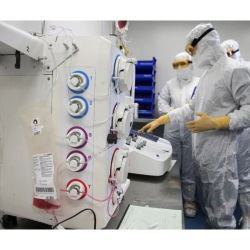
Our modern state-of-the-art manufacturing facility features a PIC/S quality system in compliance with global pharmaceutical guidelines such as ICH, USP, and EP. Our facilities are also fully compliant with major regulatory requirements including US FDA, TFDA, and EMA.
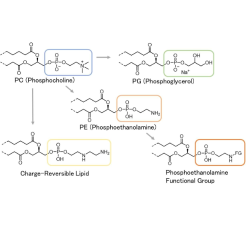
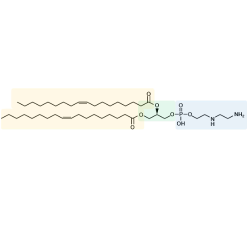
Amaran Biotech’s manufacturing capabilities include ozonolysis, thiolation, dialysis (tangential flow filtration), bio-conjugation (carbohydrate-protein conjugation), column purification (pre-HPLC, MPLC, FPLC, etc) for protein and natural production, lyophilization, etc.
【Custom Manufacturing】
- Ozonolysis
- Column Purification (Pre-HPLC, MPLC, FPLC)
- Lyophilization
- Protein and Natural Product Purification
- Carbohydrate Synthesis
- Process Scale-up and Process Validation
Other Products
Products you may be interested in
Highest Rated Products