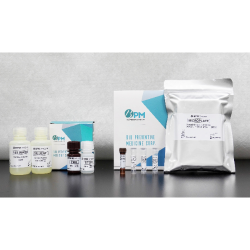
DNlite-DKD uPTM-FetA ELISA Kit
Model: DNlite-IVD103
Category: Precision Medicine
Exhibitor: BIO PREVENTIVE MEDICINE CORP.
Booth No: L418
Characteristic
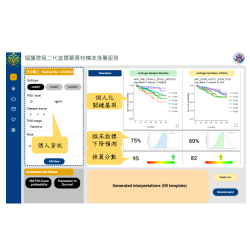
The DNlite has obtained EU IVDR certification as well as marketing approvals in multiple countries. Furthermore, the 2024 Taiwan Clinical Practice Guideline for Diabetic Kidney Disease, published by the Diabetes Association of the Republic of China (Taiwan), recommends incorporating personalized risk prediction tools, such as DNlite, on top of the existing classification system. The goal is to promote personalized care for patients with DKD in Taiwan, enabling early identification of individuals at high risk of disease progression, and providing an opportunity for early and proactive intervention to prevent advancement to end-stage renal disease.
Additionally, the 2025 AASD International Expert Consensus recommends DNlite as a personalized risk assessment tool. In alignment with international guidelines such as KDIGO, it is advised to perform risk factor reassessment every three to six months.
Products you may be interested in
Highest Rated Products