Central Lab Service
Model: Analytical services
Category: *CRO
Exhibitor: GCCL CO., LTD.
Booth No: L721
Characteristic
GCCL Central Laboratory delivers full-spectrum clinical trial sample management and analysis services with a focus on quality, scalability, and global compliance.
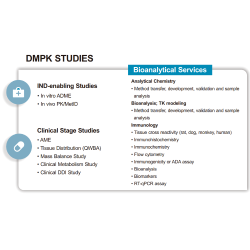
1. Comprehensive Services
- Clinical testing, biomarker profiling, safety & efficacy assessments
- PBMC isolation, DNA/RNA extraction, and sample preprocessing
- Customized Lab Kit production and sample biorepository management
2. Global Standards & Facilities
- Certified by GCLP, ISO 15189, NGSP, CAP, and ISBER
- Korea’s largest commercial central lab facility
- Multi-temperature storage: 2–8°C, -20°C, -70°C, -150°C
- Automated temperature monitoring & qualified logistics team
3) Project-Specific Management
- Dedicated project managers assigned per clinical trial
- In-house quality control program with global partner labs
- Regular inter-laboratory equivalency assessments
4) Digital Integration & Global Communication
- G-HUB: GCCL’s proprietary multilingual client portal
- Real-time tracking of sample status, results, and logistics
- Seamless communication and secure data sharing across stakeholders
Other Products
Products you may be interested in
Highest Rated Products