Clinical Operation
Model: CO
Category: *CRO
Exhibitor: EFFICIENT PHARMA MANAGEMENT CORP.
Booth No: M919
Characteristic
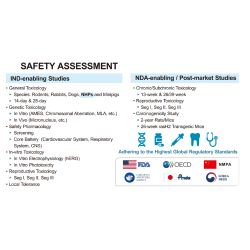
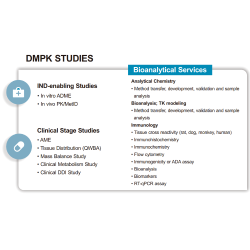
EffPha provides integrated services spanning clinical study design, regulatory services, clinical study management & monitoring, data management, and biostatistical analysis. We have extensive experience across Phase I to Phase III clinical studies, with particular expertise in early-phase oncology trials. Our cross-functional team delivers customized solutions that balance budget, timeline, and quality. Leveraging our experts’ global drug development experience and strong international partnerships, EffPha is well-positioned to support multinational clinical trials that meet international standards.
We deliver the following services:
A. Clinical Study Planning
● Site and PI Selection
● IRB Document Preparation and Submission
● Clinical Site Initiation
B. Clinical Study Management and Monitoring (Phase I-IV)
● PI Meeting
● Study Planning and Preparation
● Study Execution and Monitoring
● Health Authorities’ Inspection
● Site Audit
C. Medical Monitoring
Other Products
Products you may be interested in
Highest Rated Products