GLP/GMP One-Stop-Shop Testing Solutions for Biologics and CGT products
Category: *CRO
Exhibitor: TFBS BIOSCIENCE, INC.
Booth No: M827
Characteristic
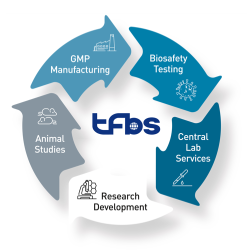
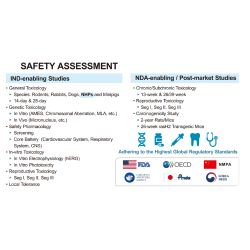
TFBS has abundant experience in a wide array of GMP/GLP testing services, including cell bank characterization, viral clearance, bulk and lot release testing, assay development in vitro and in vivo, clinical sample analysis, and cleaning validation for reusable medical devices.
We are committed to meeting the requirements of regulatory authorities worldwide, such as International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), European Pharmacopoeia (EP), and United States Pharmacopeia (USP). In the pursuit of quality, TFBS Bioscience is in full compliance with Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP) standards.
In the pursuit of quality, TFBS continuously pursues the best quality for our clients without compromise. We also comply with our clients’ requirement for cost-effective projects.
This is exactly how we help our clients and build long-term relationships with them.
Other Products
Products you may be interested in
Highest Rated Products