Scientific Evaluation & Support
Model: SE
Category: *CRO
Exhibitor: EFFICIENT PHARMA MANAGEMENT CORP.
Booth No: M919
Characteristic
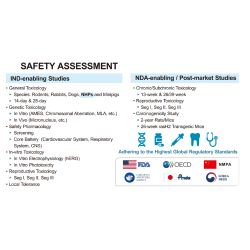
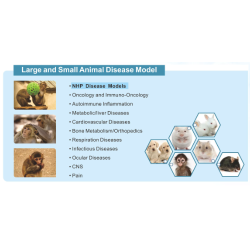
EffPha offers comprehensive risk assessment and strategic planning services for drug development. We help clients identify potential issues early in the process to ensure optimal allocation of resources throughout the development lifecycle. Our strategic consultation covers both preclinical and clinical stages, including areas such as CMC, pharmacokinetics and metabolism (PK/ADME), toxicology and safety evaluation, and statistical analysis. In addition, we support partner matching and customized sourcing to connect clients with suitable technical service providers based on their project needs.
We deliver the following services:
A. Clinical Synopsis and Protocol Design
B. Medical Writing
● Investigator's Brochure (IB)
● Informed Consent Form (ICF)
● Case Report Form (CRF)
● Clinical Study Report (CSR)
C. Strategic Consultation
D. Technical Consultation
Other Products
Products you may be interested in
Highest Rated Products