EG-Pancreatic Blood Test-E1 Pancreatic Cancer Blood Testing
Model: EG-Pancreatic Blood Test-E1
Category: Precision Medicine
Exhibitor: EG BIOMED CO., LTD.
Booth No: M710
Characteristic
Technology Overview:
EG BioMed has developed a non-invasive, high-throughput circulating cell-free DNA (cfDNA) analysis platform that integrates fully automated cfDNA extraction with high-sensitivity blood testing for pancreatic cancer. Powered by proprietary high-specificity methylation probes and quantitative PCR (qPCR) analysis, this platform leverages precise DNA methylation biomarkers to enable both early detection and longitudinal monitoring of pancreatic cancer.
The entire testing process is standardized and automated, requiring only 8 mL of peripheral blood, with results available within 2 working days. With proven high sensitivity and specificity, the test is suitable for large-scale clinical screening and post-treatment disease management. It has received a U.S. invention patent and CLIA certification (#50D2316600), making it one of the few precision testing tools currently applicable for early detection of pancreatic cancer, a disease notoriously difficult to diagnose in its early stages.
Clinical Applications:
•Early screening for high-risk individuals
Suitable for those with a family history of pancreatic cancer, chronic pancreatitis, or diabetes. The test can be included in annual health
checkups to address gaps in traditional imaging during the early stage.
•Post-surgical or treatment follow-up
Supports long-term follow-up after treatment, enabling early detection of recurrence or residual disease and improving personalized
care.
•Supportive diagnosis when imaging is inconclusive
Offers molecular-level methylation data to support more confident clinical decision-making when imaging results are unclear or
borderline.
Key Advantages:
•Non-invasive sample collection with high patient acceptance
•Fully automated workflow reduces human error and improves efficiency
•Can be tested regularly, making it useful for clinical follow-up
•U.S. patent granted and CLIA-certified lab (#50D2316600)
•Compatible with ISO15189 standards and LDT (Laboratory Developed Test) frameworks
•Clinically validated, showing high potential for commercialization and global scale-up
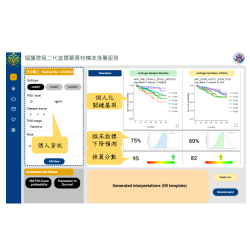
Clinical Performance:
The test has been validated through both prospective and retrospective clinical studies, showing strong performance across early-stage pancreatic cancer (Stage I–IV):
•Sensitivity: 93.8%
•Specificity: 92.9%
•Overall Accuracy: 93.3%
•Clinical cases analyzed: Over 350 subsjects
Our technology has been validated in a CLIA-certified U.S. laboratory and is currently undergoing CAP accreditation. We are also actively preparing to apply for the FDA Breakthrough Devices Program, paving the way for global clinical adoption and implementation.
Patent & Regulatory Milestones:
This technology launched its global IP strategy in 2023, beginning with priority declaration. Key patent activities include:
•Patent application filed in Taiwan
•PCT international patent application submitted
•U.S. patent granted in 2025
Additional filings are under review in various countries. This growing IP portfolio supports future clinical implementation and global commercialization.
Market Demand & Urgency:
U.S. Market (CDC data):
•Total diabetes patients in 2021: 38.4 million
•New diabetes diagnoses annually: 1.2 million
Taiwan Market (National Health Administration data):
•Total diabetes patients: Over 2 million
•New diabetes cases per year: Approx. 160,000
Research shows that 1 in every 100 newly diagnosed diabetes patients is expected to be diagnosed with pancreatic cancer within 3 years.
For newly diagnosed diabetes or chronic pancreatitis patients, the need for a reliable, early-stage pancreatic cancer blood test is urgent. This test doesn’t just complement traditional imaging—it offers a breakthrough in preventive screening and early clinical intervention.
Other Products
Products you may be interested in
Highest Rated Products