(Ezi‑Dock Systems) Next-Generation Containment: Ezi-Flow™ CSV Mk5
Model: Ezi-Flow™ CSV Mk5
Category: Pharmaceutical Equipment
Exhibitor: USIN ADVANCE CO., LTD
Booth No: M1011
Characteristic
Next-Generation Containment: Ezi-Flow™ CSV Mk5
The Ezi-Flow™ CSV Mk5 High Containment Transfer System represents the future of contained materials handling—setting a new benchmark in both performance and simplicity.
Engineered to deliver significantly lower Operator Exposure Levels (OELs) than traditional Split Butterfly Valve systems, the CSV Mk5 is ideally suited for high-potency and hazardous material transfers. This next-generation system combines exceptional containment performance with remarkable ease of use, making it the preferred choice for facilities striving to meet the most demanding safety and regulatory standards.
Key Advantages of the CSV Mk5:
OEL Performance – Achieves superior containment far beyond the capabilities of legacy split valve systems
Exceptionally Simple Operation – Streamlines operator training and minimizes process downtime
World-Class Manufacturing – Precision-engineered and assembled to the highest quality standards at Ezi-Dock’s advanced facility near Chesterfield, UK
With the Ezi-Flow™ CSV Mk5, Ezi-Dock continues to lead the way in high-containment technology—delivering solutions that are not only safer and more effective, but also more accessible and practical for modern pharmaceutical and chemical manufacturers.
Why Choose Ezi-Flow™ CSV Mk5?
Every one of the world’s top 12 pharmaceutical companies has adopted the Ezi-Flow™ system—ask yourself why.
As Active Pharmaceutical Ingredients (APIs) become increasingly potent, the need for effective containment in manufacturing processes has never been more critical. Traditional technologies, such as Split Butterfly Valves, are often rigid, costly, and insufficient in meeting the rigorous demands of current Good Manufacturing Practice (cGMP) and Operator Exposure Limits (OEL) regulations.
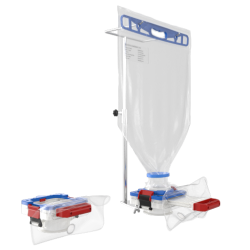
The Ezi-Flow™ CSV offers a modern, proven alternative—a unique and highly effective solution for the contained transfer of sensitive or hazardous materials. It is ideally suited for a wide range of applications, including:
Powders and solids
Tablets
Wet cake and other semi-solids
By choosing Ezi-Flow™ CSV, manufacturers benefit from enhanced containment, operational flexibility, and significant cost savings—without compromising compliance or safety.
Typical Applications
At Ezi-Dock, our products are designed with a focus on simplicity, effectiveness, and real-world usability. Developed in close collaboration with end users, Ezi-Dock solutions consistently deliver lower operational costs while outperforming many traditional technologies—bringing measurable value to daily operations.
The Ezi-Flow™ CSV system offers a complete, world-class high-containment solution ideal for a wide range of critical pharmaceutical and chemical processes, including:
Reactor Charging
Extruder Charging
Powder Filling Lines
Tablet Press Charging
Tablet Coater Charging
Blenders and Mixers
Filter Dryers
Tablet Packing Lines
Whether handling potent APIs, sensitive materials, or hazardous substances, the Ezi-Flow™ CSV ensures safe, compliant, and efficient material transfer across your manufacturing processes.
A Complete Range of Flexible Solutions
Beyond the CSV Mk5, Ezi-Dock offers a comprehensive portfolio of flexible containment and materials handling solutions designed to support the entire life science manufacturing process. From single-use components to full transfer systems, Ezi-Dock’s product range is engineered for compatibility, performance, and ease of integration.
With a strong focus on innovation, quality, and user-driven design, Ezi-Dock is proud to be a trusted partner to the global life sciences sector, delivering solutions that enhance safety, productivity, and compliance at every stage of the process.
Other Products
Products you may be interested in
Highest Rated Products