Viral Clearance
Category: *CRO
Exhibitor: CHARLES RIVER LABORATORIES
Booth No: M628
Characteristic
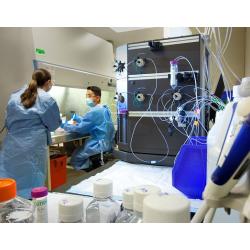
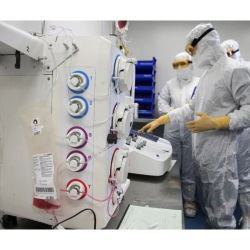
Risk-based assessments – Comprehensive review of a client’s viral safety testing program, production processes and raw material quality in order to minimize required viral clearance and ensure an economic study design
Holistic study design – Review of required pretesting, virus spikes, sampling modes, detection methods and assay sensitivities to define the most effective virus clearance study approach;
Optimized sensitivity – Standard inclusion of large volume plating for all product-relevant samples to improve LRV claims and demonstrate effective and robust virus reduction;
Customized service – Extensive experience with multiple product types and purification steps, allowing us to provide support and advice tailored to each product and its requirements, including downscale assistance, execution of process steps and interpretation and troubleshooting of results;
Industry leadership – Application of best practices as determined by performance of internal studies and as presented in multiple viral safety conferences, symposia, publications and technical reports in order to define the most scientifically sound study design.
Other Products
Products you may be interested in
Highest Rated Products