cGMP ssDNA And dsDNA Services
Category: *CRO
Exhibitor: GENSCRIPT BIOTECH
Booth No: M1023
Characteristic
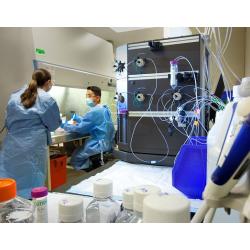
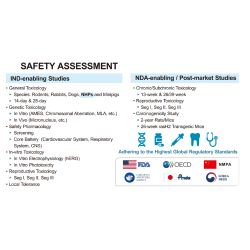
GenScript is your trusted partner for custom manufacturing of clinical-grade synthetic guide RNA (gRNA), supporting the development of non-viral gene and cell therapeutics using a variety of CRISPR gene editing systems, including Cas9, Cas12a, prime editing, base editing, and more. We offer current Good Manufacturing Practice (cGMP) manufacturing from our state-of-the-art production facility, featuring multiple dedicated cGMP production lines. Our cGMP gRNA is provided with comprehensive documentation necessary for successful IND submissions and clinical trials, including a Drug Master File (DMF) to streamline your regulatory review process.
Our standard turnaround time for cGMP gRNA is just 30 days.
Partner with GenScript to accelerate your therapeutic pipeline from early-phase research to clinical applications.
Other Products
Products you may be interested in
Highest Rated Products