Regulatory Service
Category: *CRO
Exhibitor: EFFICIENT PHARMA MANAGEMENT CORP.
Booth No: M919
Characteristic
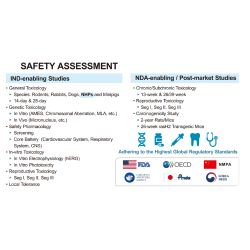
EffPha offers high-quality, comprehensive regulatory services to assist clients in meeting the regulatory requirements of various countries. Our services encompass all regulatory activities from IND to NDA/BLA stages, including Pre-IND meetings, IND submissions, EOP1 and EOP2 meetings, Pre-NDA meetings, and NDA/BLA submissions. With extensive regulatory experience and strong communication with health authorities, we effectively support our clients in achieving their submission goals. EffPha can act as the regulatory representative for clients in both Taiwan and the United States, directly communicating with regulatory agencies and submitting applications. For other countries, we collaborate with global partners to provide regulatory submission support.
We deliver the following services:
● Regulatory Gap Analysis
● Taiwan FDA/CDE Consulting Meeting Arrangement and Package Preparation
● US FDA Pre-IND Meeting Arrangement and Package Preparation
● Technical Writing/ Compilation (ICH/CTD)
● eCTD Publishing and Filing to US FDA
● IND Submission/ Maintenance
● Bridging Study Evaluation Application
● Designation for Expedited Review
● PMF Application
● NDA Submission (NDA, BLA, ANDA)
● Post-Approval Changes Application
Other Products
Products you may be interested in
Highest Rated Products