R:STEM Medium for hMSC High Growth
Model: R:STEM ( EM1-500 )
Category: Cell Therapy and Regenerative Medicine
Exhibitor: ALLISWELL BIO CO., LTD.
Booth No: L223
Characteristic
R:STEM (EM1-500) — Serum-Free, Animal-Origin-Free Culture Medium for Mesenchymal Stem Cells
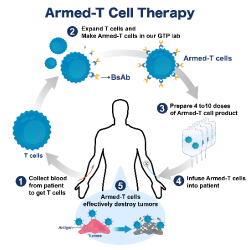
R:STEM (EM1-500) is a high-quality mesenchymal stem cell (hMSC) culture medium developed by Rohto Pharmaceutical Co., Ltd. in Japan. It is specifically formulated for hMSCs derived from adipose tissue and umbilical cord, making it ideal for cell expansion, differentiation studies, and applications in regenerative medicine.
Certifications and Quality Assurance
FIRM Mark Certification:
R:STEM (EM1-500) is the first culture medium in Japan to receive the FIRM mark based on ISO 20399 compliance.
Certified for regenerative medicine use:
Officially approved by Japan’s PMDA with a materials qualification certificate for regenerative medicine products, ensuring high standards of quality and safety.
Key Features
Serum-free and free from human or animal-derived components: Minimizes the risk of viral contamination and variability in cell characteristics.
Extracellular vesicle (EV)-free: Prevents interference from EVs present in the medium, ensuring the accuracy and reliability of experimental results.
Antibiotic-free formulation: Reduces cytotoxicity and enhances cell proliferation efficiency.
Chemically defined with minimal lot-to-lot variation: Simplifies quality control and improves experimental reproducibility.
Ready-to-use: Can be used immediately after thawing without the need for additional supplements, saving preparation time.
Experimental Validation
Cell proliferation: Supports efficient expansion of hMSCs derived from adipose and umbilical cord tissues across passages 2 to 4.
Trilineage differentiation potential: Adipose-derived hMSCs cultured in R:STEM demonstrate the ability to differentiate into adipocytes, osteocytes, and chondrocytes, confirmed by Oil Red O, Alizarin Red, and Alcian Blue staining.
Chromosomal stability: G-banding analysis confirms that hMSCs cultured with R:STEM maintain a normal karyotype.
Surface marker expression: hMSCs exhibit positive markers (CD73, CD90, CD105 >95%) and negative markers (CD11b, CD34, CD45, HLADR <2%) as defined by the International Society for Cell & Gene Therapy (ISCT).
Enhanced secretory function: R:STEM-cultured hMSCs show significantly increased secretion of extracellular vesicles (EVs) and hepatocyte growth factor (HGF).
Product Specifications
Volume: 500 mL
Storage conditions: Store frozen at ≤ -20°C
Intended use: For research use only. Not intended for diagnostic or clinical applications.
Other Products
Products you may be interested in
Highest Rated Products