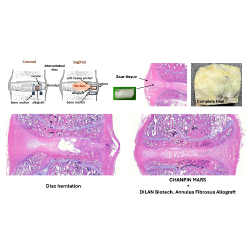
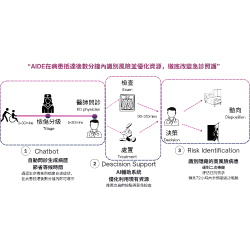
EG BioMed Multi-Cancer Blood Testing Platform
Category: Precision Medicine
Exhibitor: TAIPEI MEDICAL UNIVERSITY
Booth No: N416
Characteristic
EG BioMed has developed a high-throughput, non-invasive analysis platform for circulating cell-free DNA (cfDNA), integrating patented methylation probes with a fully automated qPCR analysis system. Only 8 mL of peripheral blood is required, and a high-sensitivity, high-specificity report can be delivered within just two working days. The procedure is non-invasive, fast, standardized, and well-accepted by patients, making it ideal for large-scale clinical implementation and long-term health monitoring.
The platform has completed technology development and clinical validation for three major cancer types, with the flexibility to expand to additional indications:
• EG-Pancreatic Blood Test-E1 Pancreatic Cancer Blood Test
• EG-Breast Blood Test-P1 Breast Cancer Follow-Up Blood Test
• EG-Colon Blood Test-E1 Colorectal Cancer Blood Test
Clinical Applications and Market Needs:
This platform addresses three key clinical scenarios:
1. Early screening for high-risk populations, including individuals with diabetes, chronic inflammation, or a family history of cancer—situations where traditional imaging often fails to detect tumors at an early stage.
2. Post-surgical or post-treatment recurrence monitoring, especially for breast and colorectal cancer patients, enabling periodic detection of residual or returning cancer.
3. A diagnostic support tool when imaging or tumor markers are inconclusive, providing DNA methylation data to strengthen clinical judgment and decision-making.
Urgent market needs include:
• Among every 100 newly diagnosed diabetic patients, 1 will develop pancreatic cancer within 3 years
• 20–30% of early-stage breast cancer patients will eventually progress to metastatic disease
• In Taiwan alone, over 16,000 new colorectal cancer cases are reported annually. One in every 13 people is at lifetime risk
These highlight the urgent clinical and screening demand for a safe, repeatable, and accurate blood test solution.
Technical Advantages:
• Blood-only testing, no invasive procedures required; high patient compliance
• Fully automated workflow with fast turnaround and reduced human error
• Suitable for repeat testing and longitudinal disease monitoring
• Built on patented probes and a standardized platform, with strong potential for global deployment
Clinical Performance:
Over 950 clinical samples have been analyzed across the three tests, demonstrating strong and consistent performance:
• EG-Pancreatic Blood Test-E1 Pancreatic Cancer Blood Test:
Sensitivity 93.8%, Specificity 92.9%, Overall Accuracy 93.3% (Sample size: >350 cases)
• EG-Breast Blood Test-P1 Breast Cancer Follow-Up Blood Test: Sensitivity 95.7%, Specificity 90.3%, Overall Accuracy 91.5% (Sample size: >500 cases)
• EG-Colon Blood Test-E1 Early-Stage Colorectal Cancer Blood Test:
Sensitivity 93.3%, Specificity 93.0%, Overall Accuracy 93.0% (Sample size: >100 cases)
These results validate the platform’s commercial readiness and clinical value.
Regulatory and IP Progress:
EG-Pancreatic Blood Test-E1 Pancreatic Cancer Blood Test
• U.S. patent granted in 2025
• Taiwan patent application and PCT submission completed (covering EU, Japan, and more)
• CLIA-certified in the U.S. (CLIA ID: 50D2316600)
• CAP accreditation and FDA Breakthrough Devices Program application in progress
• ISO 15189 compatible, deployable under LDT framework
EG-Breast Blood Test-P1 Breast Cancer Follow-Up Blood Test
• Global patent strategy initiated in 2019
• Patents granted in Taiwan (2021), the EU (2023, covering 19 countries), China and Malaysia (2024), and the U.S., Japan, Singapore, and Australia (2025)
• Additional countries are under review
• FDA De Novo submission completed
• Compatible with ISO 15189 and LDT protocols for clinical implementation
EG-Colon Blood Test-E1 Early-Stage Colorectal Cancer Blood Test
• Patent strategy launched in 2021
• Taiwan patent granted in 2023
• Applications filed in the EU, U.S., Australia, and Japan
Scalable Architecture for Multi-Cancer Applications:
This modular platform is highly expandable and can be rapidly adapted for additional cancer types, including lung, liver, and ovarian cancers. Future integration with AI analytics and personalized health platforms will enable the creation of a comprehensive, one-stop solution for cancer prevention, early detection, and ongoing monitoring. By addressing current diagnostic blind spots, this technology propels cancer screening toward a future that is earlier, broader, and more precise—forming a critical infrastructure for next-generation precision medicine.
Other Products
Products you may be interested in
Highest Rated Products