NuPlus: The First-in-Class, LNP-Encapsulated, Non-Coding Oligonucleotide Targeting DDB2 for Intravenous Treatment of Metastatic TNBC
Model: NuPlus
Category: Pharmaceutical
Exhibitor: CHINA MEDICAL UNIVERSITY
Booth No: N116
Characteristic
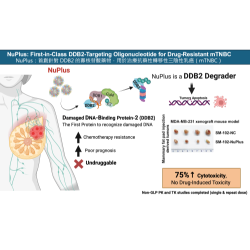
NuPlus is a first-in-class oligonucleotide therapeutic that selectively targets DDB2, the initiating protein of DNA repair. Metastatic triple-negative breast cancer (mTNBC) is a highly aggressive subtype with no approved third-line therapies. By inhibiting DDB2, NuPlus disrupts DNA repair, sensitizes tumors to chemotherapy, and effectively overcomes drug resistance and recurrence in advanced-stage patients.
NuPlus introduces a novel mechanism of action—targeting the nucleotide excision repair (NER) pathway—which has not been exploited by current oncology drugs. It is synergistic with standard chemotherapies and antibody-drug conjugates (ADCs), expanding its therapeutic versatility.
NuPlus is fully chemically synthesized, offering high cost-efficiency and manufacturing scalability. In its proof-of-concept (PoC) stage, NuPlus is formulated with FDA-approved SM102 lipid nanoparticles (LNPs) for intravenous delivery, ensuring systemic exposure and a favorable safety profile.
Non-GLP pharmacokinetic (PK) and toxicokinetic (TK) studies have been completed with no drug-related toxicities observed. Final LNP formulation selection is ongoing, and GLP studies are planned for next year.
In preclinical models, NuPlus combined with chemotherapy suppressed over 70% of tumor growth, while monotherapy achieved up to 75% tumor inhibition.
NuPlus holds multi-national patent protection, featuring traceable biomarkers, and compatible with multiple chemotherapy regimens, NuPlus is a platform-ready asset. It is ideally positioned for out-licensing, co-development, or pre-IND investment, and aligns with global pharmaceutical priorities in precision oncology, overcoming drug resistance, and advancing nucleic acid therapeutics.
Other Products
Products you may be interested in
Highest Rated Products