Jakop (Tofacitinib) Extended Release Tablet
Category: Pharmaceutical
Exhibitor: ORIENT PHARMA CO., LTD.
Booth No: M426
Characteristic
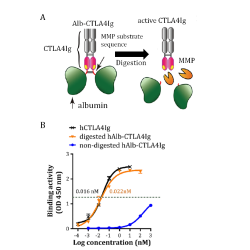
Tofacitinib OROS is a value-added formulation utilizing an Osmotic Controlled-Release Oral Delivery System (OROS), enabling steady, extended drug release. Bioequivalence studies demonstrated a perfect match to the reference listed drug (RLD), within the 80–125% range.
The global JAK inhibitor market was valued at USD 2.3 billion in 2023 and is projected to grow at a 22.6% CAGR, reaching USD 9.9 billion by 2030.
Tofacitinib OROS was submitted to the Taiwan FDA in December 2024 and completed the US FDA Paragraph IV non-infringement filing in February 2025.
Other Products
Products you may be interested in
Highest Rated Products