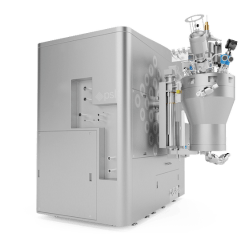
Drug Product Filtration System (DPFS)
Category: Pharmaceutical
Exhibitor: CYTIVA
Booth No: L718
Characteristic
The Drug Product Filtration System complies with EU GMP Annex 1 for sterile medicinal product manufacturing. By incorporating automated PUPSIT and in-process filter integrity testing, the system supports a robust contamination control strategy. Utilizing single-use technology, it eliminates the need for CIP/SIP cleaning validation, reducing maintenance costs and shortening batch changeover time—ultimately enhancing production efficiency.
Key Features:
* Automated filter integrity and leak testing: Fully compliant with EU GMP Annex 1 PUPSIT requirements.
* Process consistency: User-programmable methods ensure batch-to-batch reproducibility.
* Easy single-use flow path installation: Step-by-step HMI guidance and clearly labeled flow paths simplify setup and prevent installation errors.
* Flexible filter compatibility: Supports a wide range of filter sizes—from small KA1 filters to standard 10-inch filters—accommodating various process scales.
* Enhanced product recovery: Patented recovery method minimizes product loss, maximizing yield.
Other Products
Products you may be interested in
Highest Rated Products